Which of the Following Molecules Has the Highest Boiling Point
Therefore the element with the greatest total number of electrons will have the highest boiling point iodine and the element with the smallest total number of electrons will have the lowest boiling point hydrogen. Which of the following organic compounds has the highest boiling point.

Intermolecular Forces How Can I Determine The Highest Boiling Point Given A List Of Molecules Chemistry Stack Exchange
1 lowest CH3CH2OCH3 CH3CH2OH CH3CH2CH2OH highest.

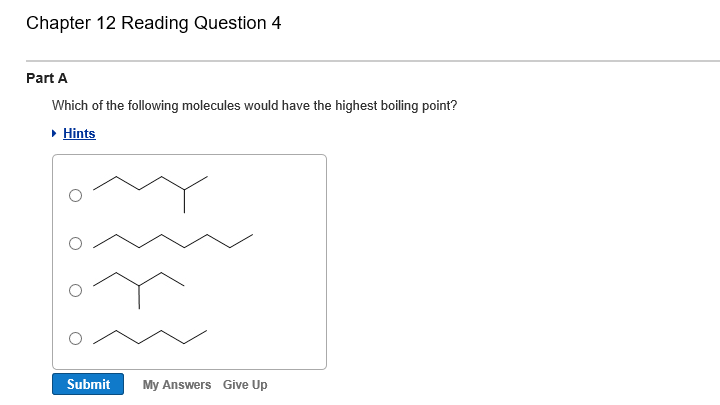
. Pentanol has a higher boiling point compared to others because it contains stronger intermolecular interactions known as. Of the two CH3CH2CH2OH has more electrons and thus stronger London forces so it is the higher-boiling compound. Octane will have a higher boiling point than 2233tetramethylbutane because it branches less than 2233tetramethylbutane and therefore has a larger surface area and more van der Waals forces.
1-chlorobutane has a higher boiling point among the given compounds. Go through the list above. To predict boiling point we should have idea about van der waals forces which depend on surface area of the given mole View the full answer Transcribed image text.
N-hexane n-pentane 2-methylbutane 2 2-dimethylpropane. Next comes methanol CH4O or CH3OH. Bigger molecules will have stronger London dispersion forces.
Ammonia NH3 has the highest boiling point at -3334 C while Methane CH4 has a boiling point of -1616 C and Hydrogen sulfide SH2 has a boiling point of -60 C. The boiling points of the given compounds are as follows 1 2-Pentanone 101 C. Isopropyl Chloride 1-chloropropane 1-chlorobutane.
What is the correct relation between acidic strength of primary secondary and tertiary alcohols. Among given compound 2 n-hexane is a straight chain molecule and higher molecular mass thus it has the highest boiling point. Of the diatomic elements H2 N2 O2 F2 Cl2 Br2 I2 all have dispersion forces.
Further the boiling point of these compounds follows the order. Stereoisomers formed by ring formation at the carbon which was originally a carbonyl aldehyde or ketone in the open chain form of monosaccharides. CH3OH hydrogen bonding.
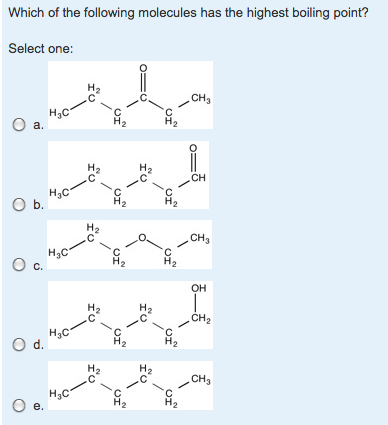
Dimethyl ether CH3OCH3 is a polar molecule. 1-Pentanol has the highest boiling point. None of these have dipoles.
Which of the following molecules has the highest boiling point. Asked Sep 1 2019 in Chemistry by MangaLover general-chemistry. CH4 is non-polar hence contain only weak van der waals forces hence it will have a low boiling point.
CaCO3 is an ionic compound. THIS IS THE BEST ANSWER. The Correct Answer is A 1-chlorobutane.
CH3CH2CH344 amu 01 dipole moment CH3OCH346 amu 13 dipole moment CH3Cl50 amu 19 dipole moment CH3CHO44 amu 27 dipole moment CH3CN41 amu 39 dipole moment. This is because Ammonia can form strong hydrogen bonds but which makes it. Water boils at 100C.
This is because intermolecular forces often called van der Waals forces depend on attractions caused by quantum fluctuations in. Which of the following molecules bartleby. 4 Painting 103 C.
Which of the following molecules has the highest boiling point. HCl will have a lower boiling point than HF since F is more electronegative than Cl and possess a greater degree of hydrogen bonding. Which of the following organic bartleby.
Methanol has strong hydrogen bonds. CO2 is a nonpolar molecule hence it has the lowest boiling point. 2 - Methyl pentane has the highest boiling point because the boiling point in alkane depends upon the chains of atoms and the molecular mass of the molecules.
List the following molecules in order of increasing boiling point. The boiling point of 1-chlorobutane is 785C. 3 Painting 361 C.
Nitrogen is more electronegative than sulphur hence NH3 is more polar than H2S and consequently NH3 has a higher boiling point than H2S due to stronger dipole-dipole interaction. Which of the following molecules has the highest boiling point AnswerHF will have the highest boiling pointExplanationCH4 is non-polar hence contain only weak van der waals forces hence it will have a low boiling point. HCl will have a.
Higher molecular mass molecules have higher boiling point than the lower mass molecules. The hydrogen bonding in CH3CH2OH and CH3CH2CH2OH give these molecules a higher boiling point. Alcohol are found to have boiling point approximately as 7837 C.
P is much less electronegative than both Cl and F hence PH3 has a much. None of these have hydrogen bonding. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule.
It has the highest boiling points. Only weak dispersion forces exist in CO2. Sodium chloride has boiling point 1413 C.
So it is easy to identify that NaCl sodium chloride has highest boiling point amongst the given choices. The order of the boiling points are. 2 1-Pentanol 138 C.
Straight-chain molecules have higher boiling point than the branched-chain compounds. Which of the following has the highest boiling point. CH3OCH3 dipole-dipole attractions.
Which of the following has the highest boiling point. Toluene and phenol have similar boiling points. Br2 F2 I2 Cl2 Answer Higher boiling points will correspond to stronger intermolecular forces.
Propan-1-ol boils at a higher temperature than propan-2-ol. The boiling point of 1-chloropropane is 467C. AnswerHF will have the highest boiling point.
Of the two molecules shown in the figure one would expect that CO2 has a higher boiling point than NO2 because CO2 is linear and nonpolar. QUESTION 10 Which of the following molecules has the HIGHEST boiling point. CH4 London dispersion forces.
It will have the next highest boiling point. Glucose usually melts at 146 C. Nonane will have a higher boiling point than octane because it has a longer carbon chain than octane.
Butanoic acid OH O 22-dimethylbutane Pentanol Ethyl ethanoate.
Solved Which Of The Following Molecules Would Have The Chegg Com

Answered 16 Which Of The Following Molecules Bartleby
Solved Which Of The Following Molecules Has The Highest Chegg Com

Comments
Post a Comment